Researchers reveal how humans could regenerate lost body parts
Bone regeneration is a remarkable process that varies widely across species and presents significant opportunities for advancing human medicine.
For decades, scientists have focused on how certain animals like salamanders and zebrafish regenerate complex structures, hoping to uncover clues that could one day lead to breakthroughs in human limb regeneration.
While the mechanisms behind regeneration have long been studied, new research challenges old assumptions and brings fresh insights into the critical role of mechanical load in bone regeneration.
In mammals, bone repair typically occurs through processes like fracture healing and distraction osteogenesis. Fracture healing involves the formation of a callus by periosteal cells to bridge the gap. However, this repair mechanism has limits. Large fractures often fail to heal, leading to non-union.

Distraction osteogenesis, a surgical technique, stimulates bone growth by applying mechanical load across a fracture site, promoting repair and extending bone length. Yet another fascinating form of bone repair, less studied but equally illuminating, is the regeneration of the digit tip after amputation. This process occurs in both rodents and humans, demonstrating the potential for bone regeneration that goes beyond standard repair mechanisms.
Digit tip regeneration exemplifies a unique biological phenomenon where amputated bone autonomously grows to restore its original structure. This form of de novo bone formation replaces lost bone entirely, unlike the bridging of injured tissues seen in fracture repair.
Regeneration begins with the amputation of the digit tip, which transects various tissue types, including nail, epidermis, central bone, dermis, nerves, and blood vessels. The initial wound healing triggers an inflammatory response, leading to the recruitment of monocytes that differentiate into osteoclasts.
These osteoclasts initiate an extended phase of bone resorption, reducing the bone volume by an additional 40% to 50% beyond the amputation’s original loss of 10% to 20%.
As the resorption phase concludes, a blastema—a mass of proliferating, heterogeneous cells—forms at the site. This hallmark of epimorphic regeneration contrasts with tissue-specific repair or fibrotic healing.
Progenitor cells differentiate into osteoblasts through intramembranous ossification, sequentially rebuilding the bone from the proximal to distal ends. This highly reproducible process provides a model for studying bone regeneration mechanisms in mammals.
Emerging research has revealed parallels between digit tip regeneration and skeletal development during growth. For example, reduced mechanical load during development leads to diminished bone mass, underscoring the importance of physical forces in shaping the skeleton.
Mechanical load is equally vital in fracture repair and distraction osteogenesis. In salamanders and zebrafish, two common models for studying limb and fin regeneration, the absence of mechanical load significantly impairs regeneration.
However, the role of mechanical loading in mammalian digit tip regeneration remained unclear until recent studies by Dr. Ken Muneoka and his team challenged long-held assumptions.
Dr. Muneoka, a professor at Texas A&M University, has been at the forefront of regeneration research. In a groundbreaking 2019 study, his team demonstrated joint regeneration in mammals, breaking new ground in the field.
More recently, his research has upended a two-century-old belief that nerves are essential for regeneration. Instead, his findings highlight the crucial role of mechanical load. These discoveries mark a paradigm shift in how scientists view the requirements for mammalian regeneration and its potential applications in human medicine.
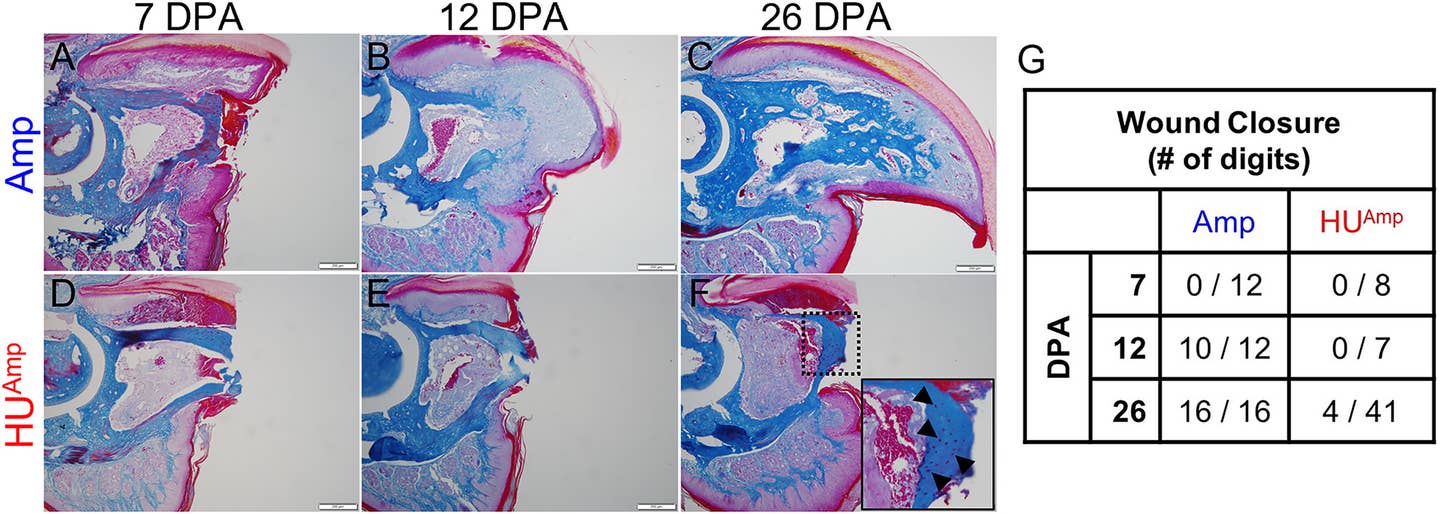
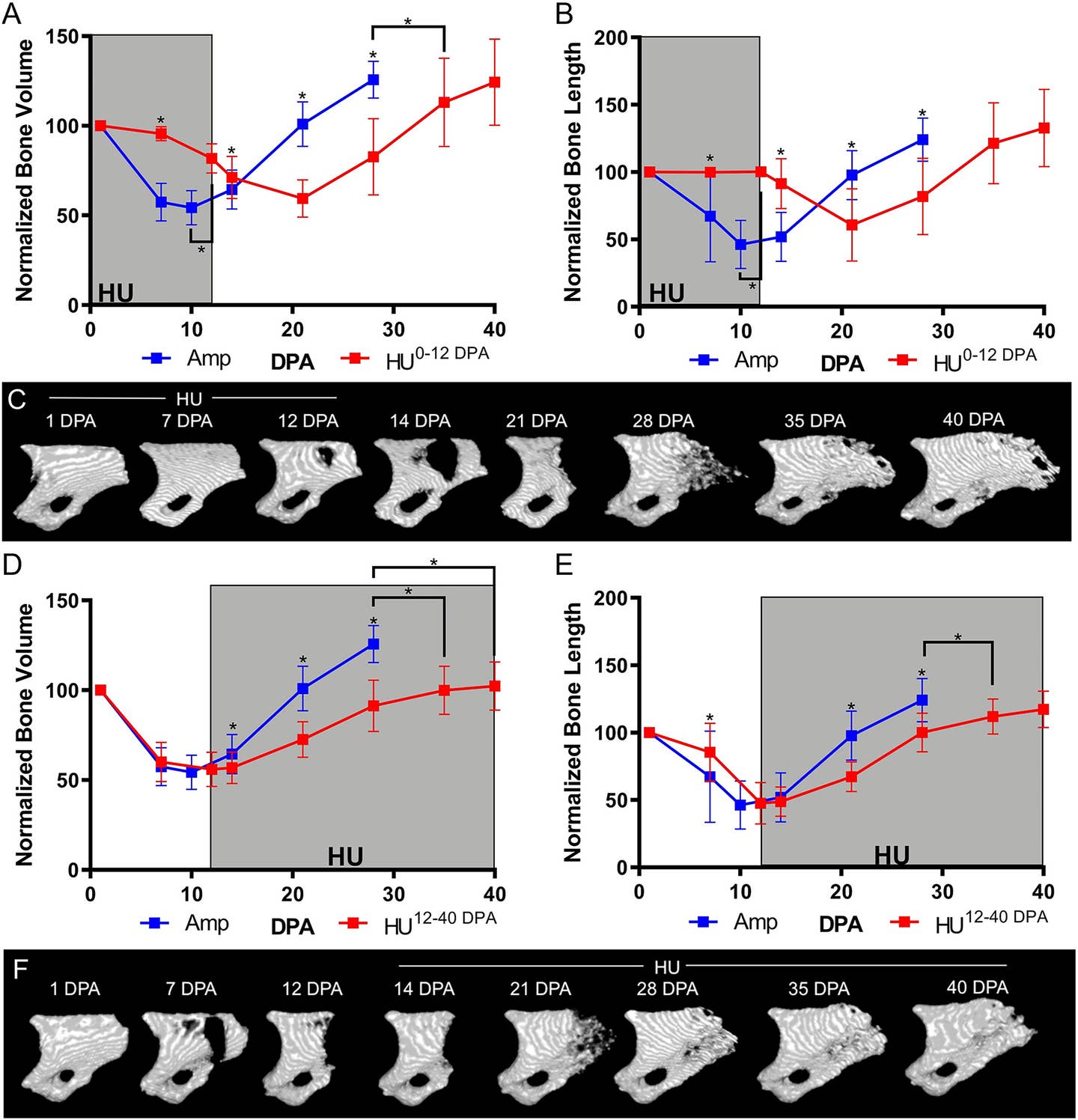
One of the studies, published in the Journal of Bone and Mineral Research, used a hindlimb unloading (HU) model to explore the effects of mechanical loading on digit tip regeneration. In this model, hindlimbs are suspended to eliminate weight-bearing forces, mimicking conditions of zero gravity.
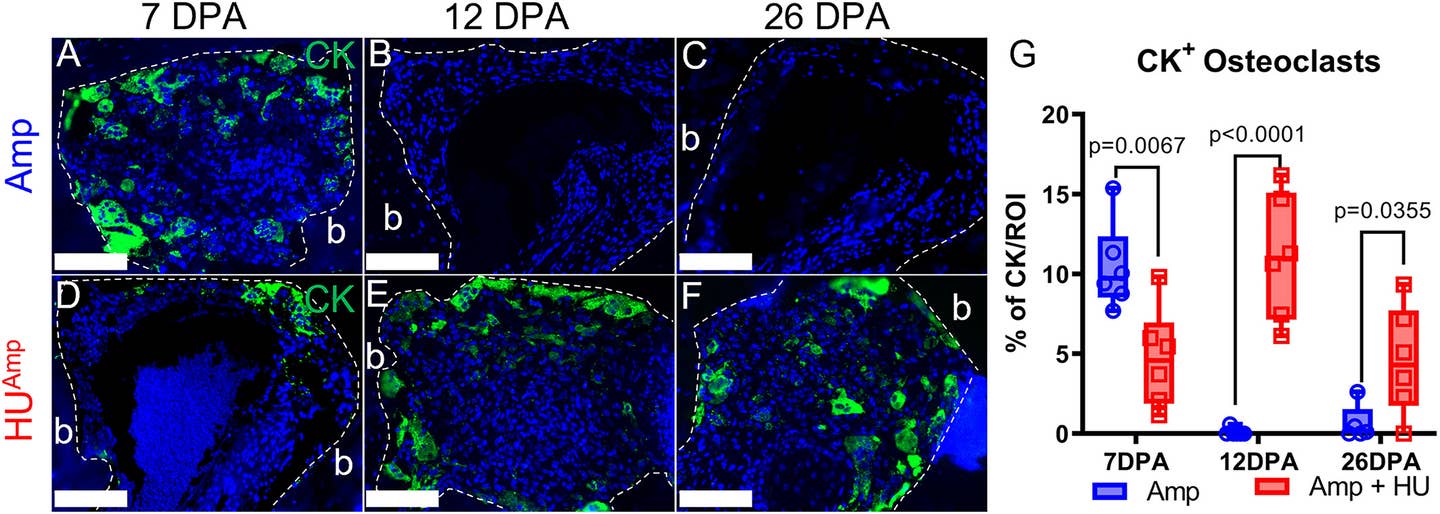
Connor Dolan, the study’s first author, drew inspiration from NASA’s research on the effects of microgravity on bone. The results were striking: regeneration halted entirely in non-weight-bearing limbs, despite the presence of nerves. Once mechanical load was restored, regeneration resumed, albeit with a delay.
“Absolutely nothing happens during the suspension,” Dr. Muneoka explained. “But once the load returns, regeneration begins after a short delay.” These findings establish mechanical load as a critical factor for regeneration, independent of nerve presence.
A subsequent study, published in Developmental Biology, demonstrated that even denervated digits could regenerate under mechanical load. This discovery contradicts long-standing dogma and redefines the role of nerves in mammalian regeneration.
The implications of these studies extend beyond academic debates. For decades, researchers have focused on the role of nerves and growth factors in regeneration. While these factors remain important, mechanical load introduces a new dimension to the puzzle.
Dr. Muneoka’s findings suggest that nerves are not a prerequisite for regeneration but rather one of several components that must be addressed. From his perspective, the goal shifts from relying on nerves to considering them as part of what needs to be regenerated.
Dr. Larry Suva, head of the Department of Veterinary Physiology and Pharmacology at Texas A&M, emphasized the importance of this shift. “Think of a blast injury where a soldier is left with a stump,” he said. “No one was even considering the mechanical influences before this paper.”
The new findings compel scientists to reevaluate their approaches to regeneration, integrating mechanical load into the equation.
Mechanical load impacts cells at a biochemical level, translating physical forces into cellular signals that drive regeneration. While some researchers have studied the biochemical basis of mechanical load, much remains to be understood.
Dr. Muneoka’s work suggests that mimicking these signals could one day replace the need for physical force, opening new possibilities for regenerative medicine. By developing molecular cocktails that replicate the effects of mechanical load, scientists could advance closer to achieving human limb regeneration.
While regenerating entire human limbs remains a distant goal, these discoveries mark a significant milestone on the path toward that future. Dr. Suva described the findings as a “major marker” in the journey toward full human regeneration.
“Regeneration of a human limb may still be science fiction,” he said, “but we now know mechanical load is as essential as growth factors. That changes how future scientists and engineers will tackle this challenge.”
These breakthroughs also highlight the value of challenging established beliefs. For years, regeneration research has been guided by studies on salamanders and other species, whose mechanisms differ significantly from those of mammals.
Dr. Muneoka’s work reminds the scientific community to consider species-specific differences and adopt a broader perspective. By integrating mechanical load into regeneration studies, researchers can address previously overlooked aspects of the process, paving the way for innovative solutions.
The road to human regeneration is long and complex, but with each discovery, the path becomes clearer. Dr. Muneoka’s research underscores the importance of mechanical load and offers new insights into the factors that drive regeneration.
As scientists continue to build on these findings, the dream of regenerating human limbs moves closer to reality. While many challenges remain, the future of regenerative medicine holds immense promise, thanks to the pioneering work of researchers like Dr. Muneoka and his team.
Source link
